Monocyte isolation for maturation of dendritic cells with pluribead®
Isolation
| Sample material | 10 ml human buffy coat |
| Isolation method | Non-magnetic with 280µl CD14 S-pluriBead® anti-hu or 300µl M-pluriBead® anti-hu |
| Yield | ~6 * 10^6 S-pluriBead® or ~12 * 10^6 M-pluriBead® |
| Vitality | >92% (Trypan blue staining) |
| Purity | ~97% |
Monocyte isolation, differentiation and stimulation protocol
Wash sample material twice with provided Washing Buffer (PBS + 0,05 % BSA and 2 mM EDTA, pH 7,4) in order to reduce soluble CD14. Add pluriBead CD14 for isolation of monocytes into the sample tube and incubate on a pluriPlix or wiping rolling mixer for 20 minutes.
After isolation, resuspend the cells in 1ml of RPMI 1640-medium (+10 % FCS and 1x Pen/Strep) and determine the cell number. For the cultivation of monocytes in a 24-well cell culture plate, 1 million cells per well are used and incubated with 1ml monocytes-culture-medium (RPMI 1640 + 10 % FCS, 1x Pen/Strep, 2000 U/ml GM-CSF und 200 U/ml IL-4) at 37 °C and 5 % carbon dioxid.
After 24h, remove the medium and add 1ml fresh monocytes-culture-medium. Then, the cells are cultured for 4 more days. After a total of 5 days, stimulate the cells with 100ng/ml LPS for another 24h. The activated cells are then removed with trypsin and the maturation rate to dentritic cells from monocytes is detected by fluorescent-analysis of CD1a, CD14 and CD83.
Data
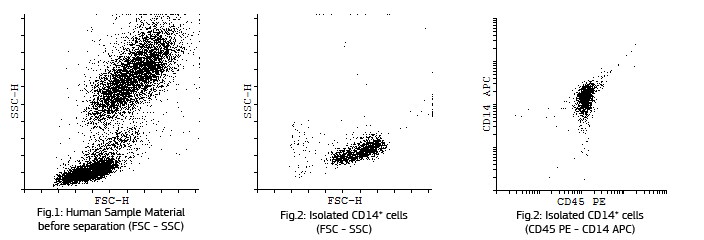
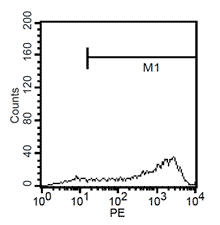
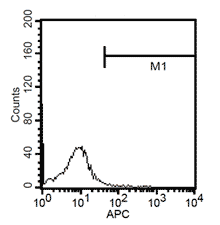
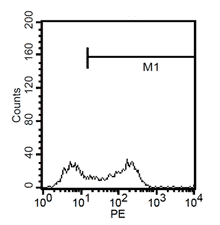
Fluorescent analysis of mature dentritic cells CD11a+, CD14+, CD83+
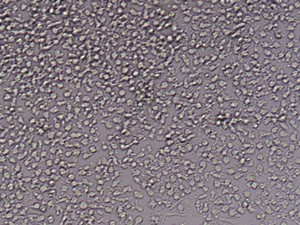
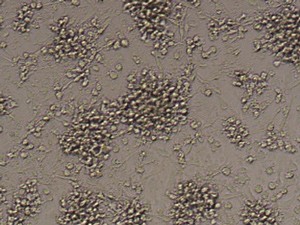
CD14+ cells after isolation in culture, Maturation of monocytes to dendritic cells after 5 days of culture with GM-CSIF/IL-4 and stimulation with LPS
 English
English French
French
 German
German
 Spanish
Spanish
 Belgium
Belgium
 Italian
Italian Brazil
Brazil Chinese (Simplified)
Chinese (Simplified)